Microbiology Lab Rules And Regulations . Potential hazards in the microbiology laboratory have been identified and explained. Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. Hsa has started regulatory control of cpm products under the medicines act since september 1999. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. You understand that the bacterial cultures used in. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Personnel should be made aware of: The following laboratory rules and regulations should be adhered to at all times, no exceptions.
from www.medlabmag.com
Hsa has started regulatory control of cpm products under the medicines act since september 1999. Personnel should be made aware of: This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. The following laboratory rules and regulations should be adhered to at all times, no exceptions. Potential hazards in the microbiology laboratory have been identified and explained. You understand that the bacterial cultures used in. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3.
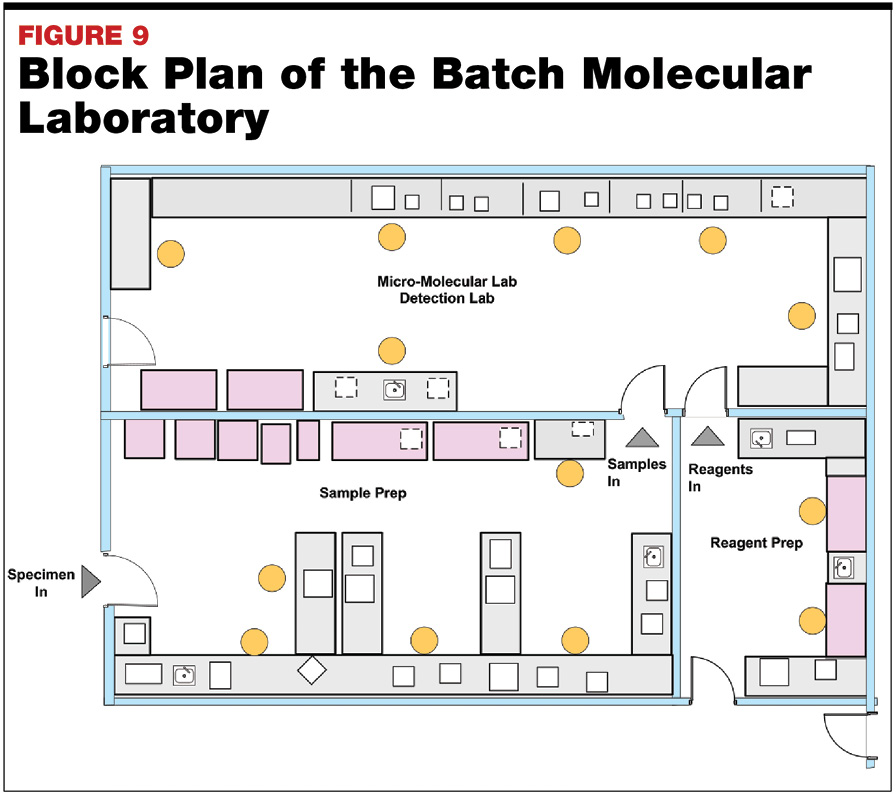
Modern Microbiology Laboratory Planning and Design May 2019
Microbiology Lab Rules And Regulations 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. You understand that the bacterial cultures used in. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. Potential hazards in the microbiology laboratory have been identified and explained. Personnel should be made aware of: This document outlines a comprehensive practical approach to a laboratory quality management system (qms). 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Hsa has started regulatory control of cpm products under the medicines act since september 1999. The following laboratory rules and regulations should be adhered to at all times, no exceptions.
From www.laboratorydeal.com
Microbiological Lab Equipment list Laboratory Equipment laboratorydeal Microbiology Lab Rules And Regulations Personnel should be made aware of: Hsa has started regulatory control of cpm products under the medicines act since september 1999. The following laboratory rules and regulations should be adhered to at all times, no exceptions. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. You understand that the bacterial. Microbiology Lab Rules And Regulations.
From www.youtube.com
Signs and symbols use in Microbiology lab Safety signs in lab YouTube Microbiology Lab Rules And Regulations This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Hsa has started regulatory control of cpm products under the medicines act since september 1999. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. You understand that the bacterial cultures used in. Personnel should be made. Microbiology Lab Rules And Regulations.
From www.youtube.com
Basics of Microbiology Lab Safety YouTube Microbiology Lab Rules And Regulations Hsa has started regulatory control of cpm products under the medicines act since september 1999. Personnel should be made aware of: Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). The following laboratory rules and regulations. Microbiology Lab Rules And Regulations.
From www.medlabmag.com
Modern Microbiology Laboratory Planning and Design May 2019 Microbiology Lab Rules And Regulations The following laboratory rules and regulations should be adhered to at all times, no exceptions. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. This document outlines a comprehensive practical approach to a laboratory quality management system. Microbiology Lab Rules And Regulations.
From studylib.net
Microbiology Laboratory Safety Guide 1). Objectives Microbiology Lab Rules And Regulations Hsa has started regulatory control of cpm products under the medicines act since september 1999. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Potential hazards in the microbiology laboratory have been identified and explained. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. Procedures. Microbiology Lab Rules And Regulations.
From sciencenotes.org
Lab Safety Rules and Guidelines Microbiology Lab Rules And Regulations Personnel should be made aware of: Potential hazards in the microbiology laboratory have been identified and explained. Hsa has started regulatory control of cpm products under the medicines act since september 1999. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). You understand that the bacterial cultures used in. Procedures or manipulations involving potentially infectious. Microbiology Lab Rules And Regulations.
From www.studocu.com
Microbiology Activity 1 Safety in the Laboratory General Rules inside Microbiology Lab Rules And Regulations 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Potential hazards in the microbiology laboratory have been identified and explained. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. Section 3 core. Microbiology Lab Rules And Regulations.
From www.medlabmag.com
Modern Microbiology Laboratory Planning and Design May 2019 Microbiology Lab Rules And Regulations This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. Hsa has started regulatory control of cpm products under the medicines act since september 1999. Potential hazards in the microbiology laboratory have been identified and explained.. Microbiology Lab Rules And Regulations.
From covotsosbiology.weebly.com
Safety in the Biology Laboratory Microbiology Lab Rules And Regulations Hsa has started regulatory control of cpm products under the medicines act since september 1999. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). You understand that the bacterial cultures used in. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. Procedures or manipulations involving. Microbiology Lab Rules And Regulations.
From studylib.net
Safety in the Clinical Microbiology Laboratory Microbiology Lab Rules And Regulations 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Hsa has started regulatory control of cpm products under the medicines act since september 1999. Potential hazards in the microbiology laboratory have been identified and explained. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. This document outlines. Microbiology Lab Rules And Regulations.
From www.futurelearn.com
The microbiology lab and AMS Microbiology Lab Rules And Regulations This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Potential hazards in the microbiology laboratory have been identified and explained. Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. The following laboratory rules and regulations should be adhered to at all times, no exceptions.. Microbiology Lab Rules And Regulations.
From studylib.net
Microbiology Lab Manual Microbiology Lab Rules And Regulations Personnel should be made aware of: You understand that the bacterial cultures used in. Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Hsa has started regulatory control of cpm products under the medicines act since september. Microbiology Lab Rules And Regulations.
From eleanorjcarrollxo.blob.core.windows.net
Micro Lab Calicut Microbiology Lab Rules And Regulations Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Hsa has started regulatory control of cpm products under the medicines act since september 1999. The following laboratory rules and regulations should be adhered to at. Microbiology Lab Rules And Regulations.
From materialmediamatisse.z14.web.core.windows.net
Laboratory Rules And Safety Microbiology Lab Rules And Regulations Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. Potential hazards in the microbiology laboratory have been identified and explained. Hsa has started regulatory control of cpm products under the medicines act since september 1999. Personnel should be made aware of: The following laboratory rules and regulations should be adhered. Microbiology Lab Rules And Regulations.
From www.studocu.com
Microbiology Lab Safety PRELAB QUESTIONS What constitutes personal Microbiology Lab Rules And Regulations This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Potential hazards in the microbiology laboratory have been identified and explained. You understand that the bacterial cultures used in. 2.1.5 access to the microbiological laboratory should be restricted to authorized personnel. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence. Microbiology Lab Rules And Regulations.
From mungfali.com
Designing A Microbiology Laboratory D90 Microbiology Lab Rules And Regulations You understand that the bacterial cultures used in. Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples) are to be carried out in a certified. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. This document outlines a comprehensive practical approach to a laboratory quality management system. Microbiology Lab Rules And Regulations.
From www.safepointapp.com
A guide to lab safety (free poster!) Safepoint Lone worker apps and Microbiology Lab Rules And Regulations The following laboratory rules and regulations should be adhered to at all times, no exceptions. Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Procedures or manipulations involving potentially infectious materials (e.g., handling of clinical samples). Microbiology Lab Rules And Regulations.
From echamicrobiology.com
Laboratory Analysis Testing Services ECHA Microbiology Microbiology Lab Rules And Regulations Section 3 core requirements 27 3.1 good microbiological practice and procedure 27 3.2 personnel competence and training 31 3.3. This document outlines a comprehensive practical approach to a laboratory quality management system (qms). Personnel should be made aware of: The following laboratory rules and regulations should be adhered to at all times, no exceptions. 2.1.5 access to the microbiological laboratory. Microbiology Lab Rules And Regulations.